Adhesion and Cohesion
 If you haven’t, read my “Hydrogen bonds” blog first if
possible. Like I said, this time we are going adhesion and cohesion. Let’s
start with adhesion. Adhesion are 2 materials (example: water and glass) that
have different polarities and can stick to each other.
If you haven’t, read my “Hydrogen bonds” blog first if
possible. Like I said, this time we are going adhesion and cohesion. Let’s
start with adhesion. Adhesion are 2 materials (example: water and glass) that
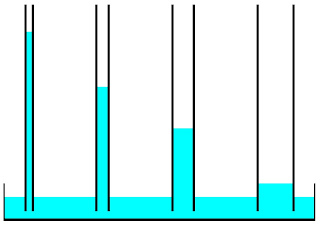
have different polarities and can stick to each other.One interesting thing about adhesion is the capillary action that makes water climb up a glass tube. Now you need to imagine. Imagine that you have a plastic tub. Then you have a very thin glass rod, about 3 mm thick, and it’s hollow. You put the glass rod in the tub, and something interesting happens.
The water will climb up the glass tube. It climbs up the glass tube because it’s more attracted to the glass than to itself. This is also adhesion because it is attracted to each other. They are attracted because water has a polarity and glass also has one.
Adhesion happens because there are polarities in some atoms, like water and glass. A positive atom will stick to a negative atom. Same goes for cohesion. But, cohesion is a little different.
Cohesion is when a material is attracted to itself, such as water. Water is H2O, and as you probably already know, H stands for hydrogen, and O stands for oxygen.
Oxygen has a partially positive polarity and hydrogen has a partially negative bond. 2 water molecules can bump and form a hydrogen bond. I explained in in my blog “Hydrogen bonds.” Polarities are why adhesion and cohesion happen.
Water is very interesting. There is still a lot to learn. I’m going to explain it in future blogs. I’m thinking that I should add acidity too!
Thank you! - Nat



Comments
Post a Comment