Adhesion and Cohesion
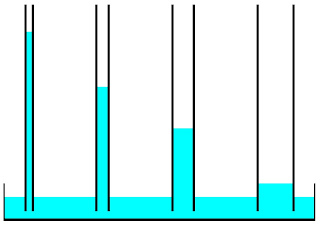
If you haven’t, read my “Hydrogen bonds” blog first if possible. Like I said, this time we are going adhesion and cohesion. Let’s start with adhesion. Adhesion are 2 materials (example: water and glass) that have different polarities and can stick to each other. One interesting thing about adhesion is the capillary action that makes water climb up a glass tube. Now you need to imagine. Imagine that you have a plastic tub. Then you have a very thin glass rod, about 3 mm thick, and it’s hollow. You put the glass rod in the tub, and something interesting happens. The water will climb up the glass tube. It climbs up the glass tube because it’s more attracted to the glass than to itself. This is also adhesion because it is attracted to each other. They are attracted because water has a polarity and glass also has one. Adhesion happens because there are polarities in some atoms, like water and glass. A positive atom will stick to a negative atom. Same goes for cohesion. But, cohe...


